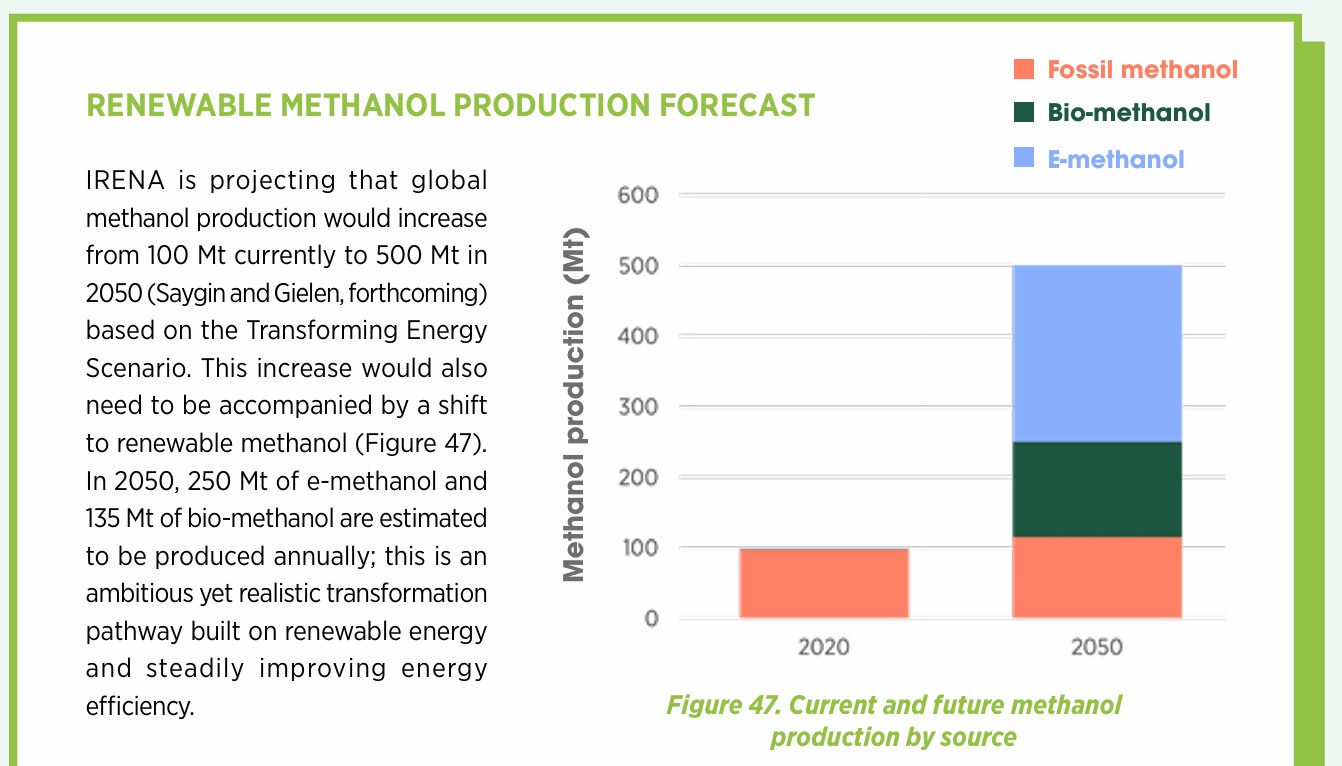
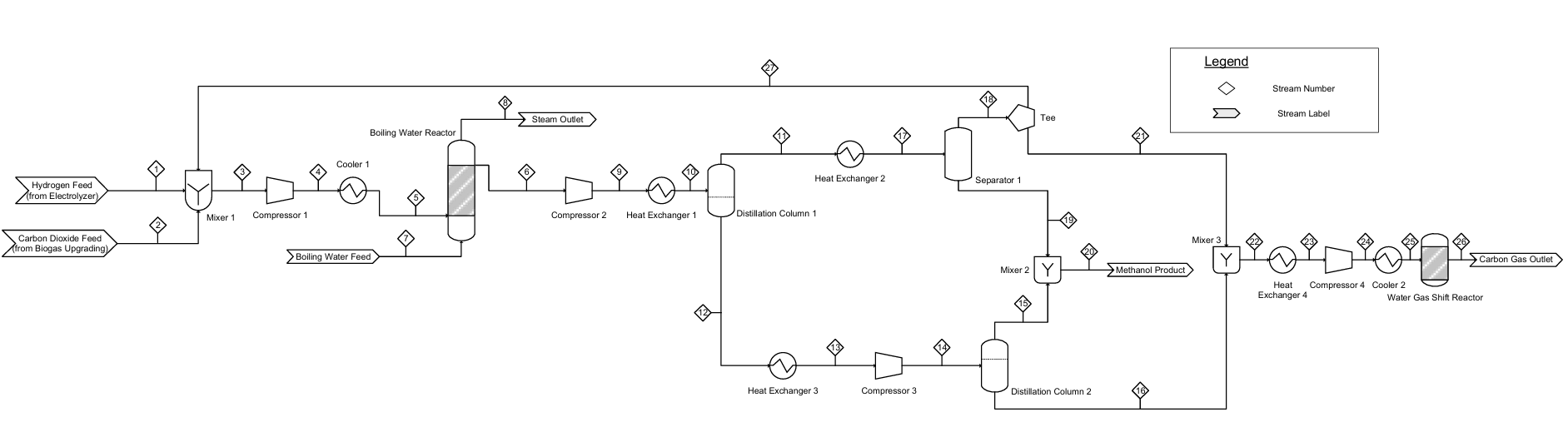
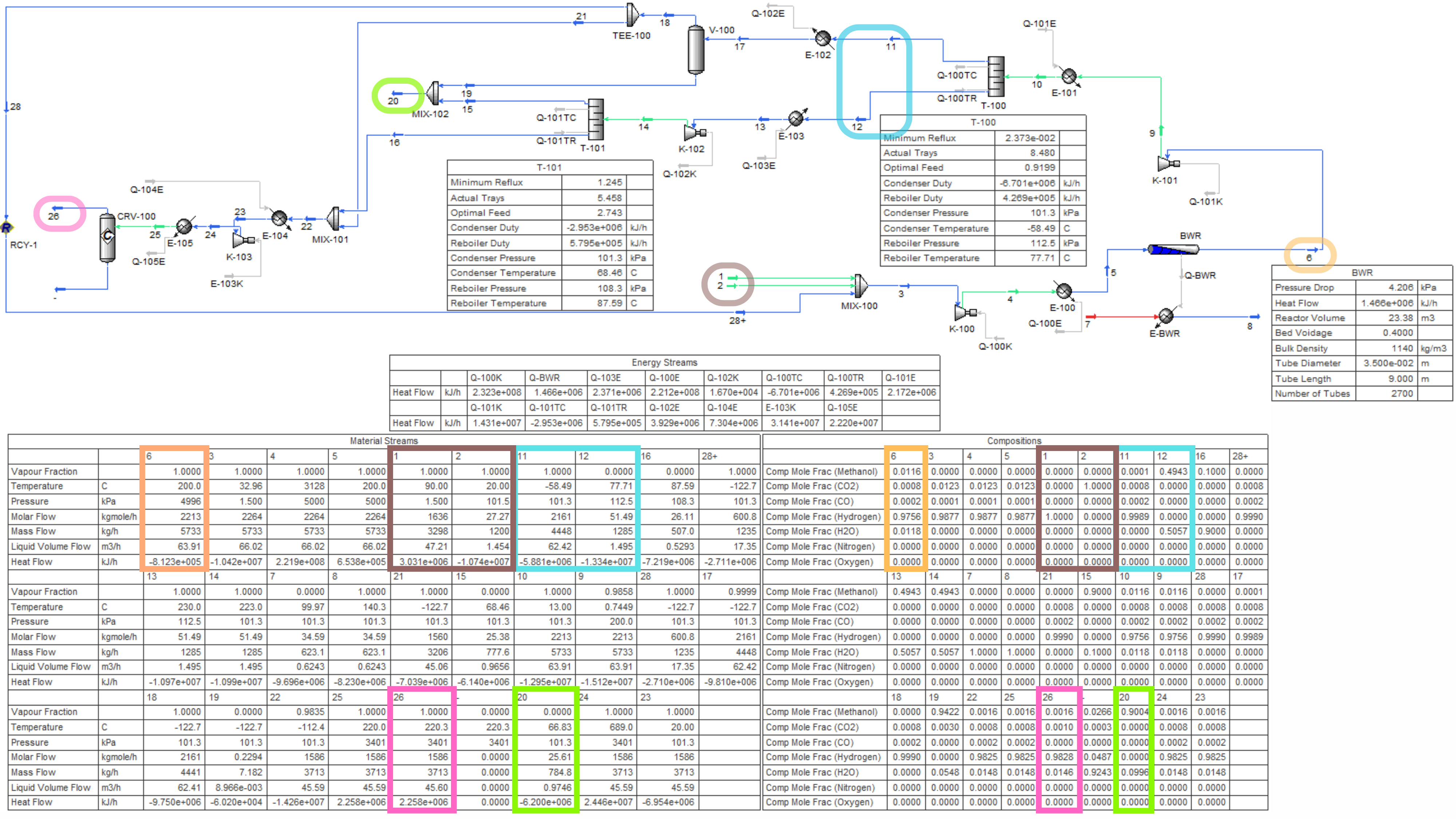
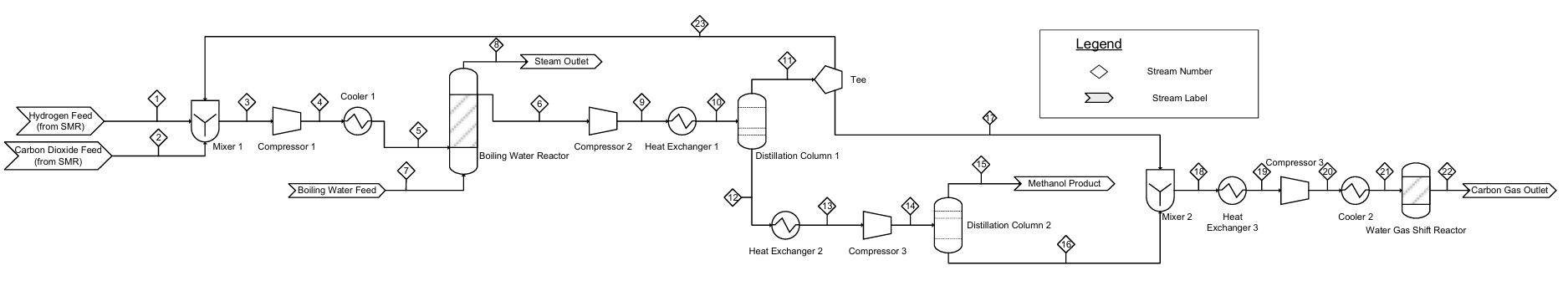
Contributors
please click on names or images for LinkedIn

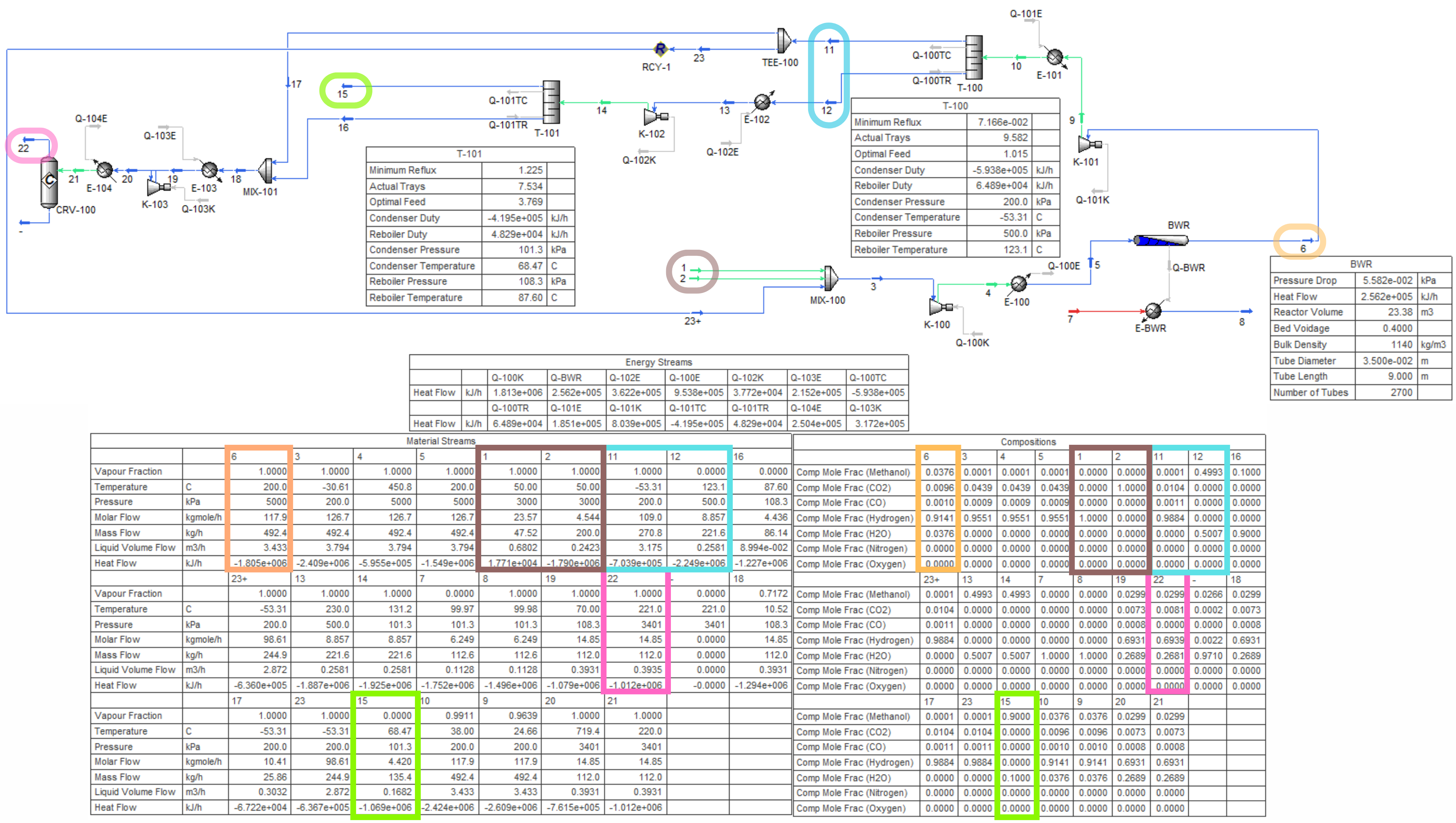
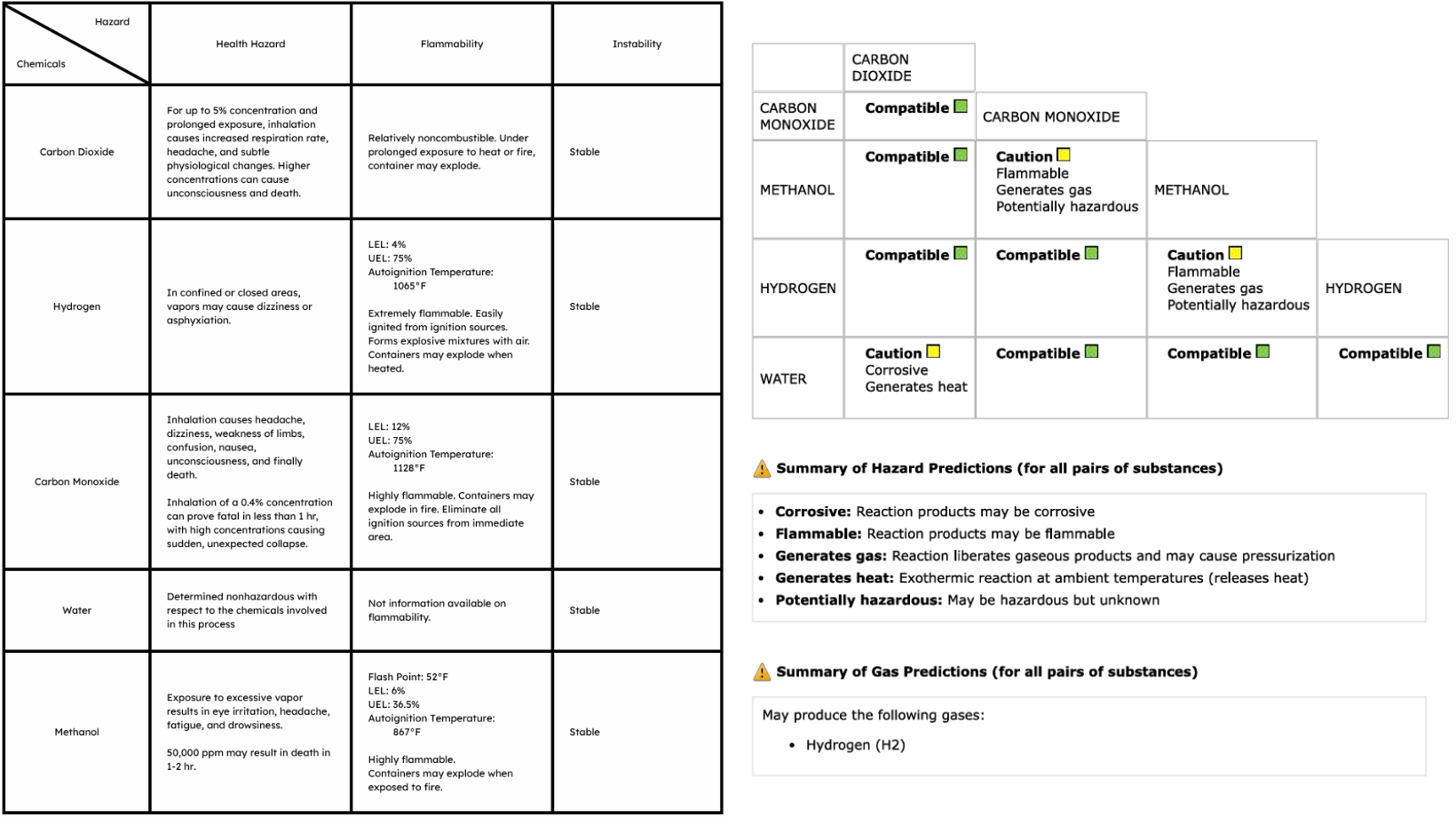
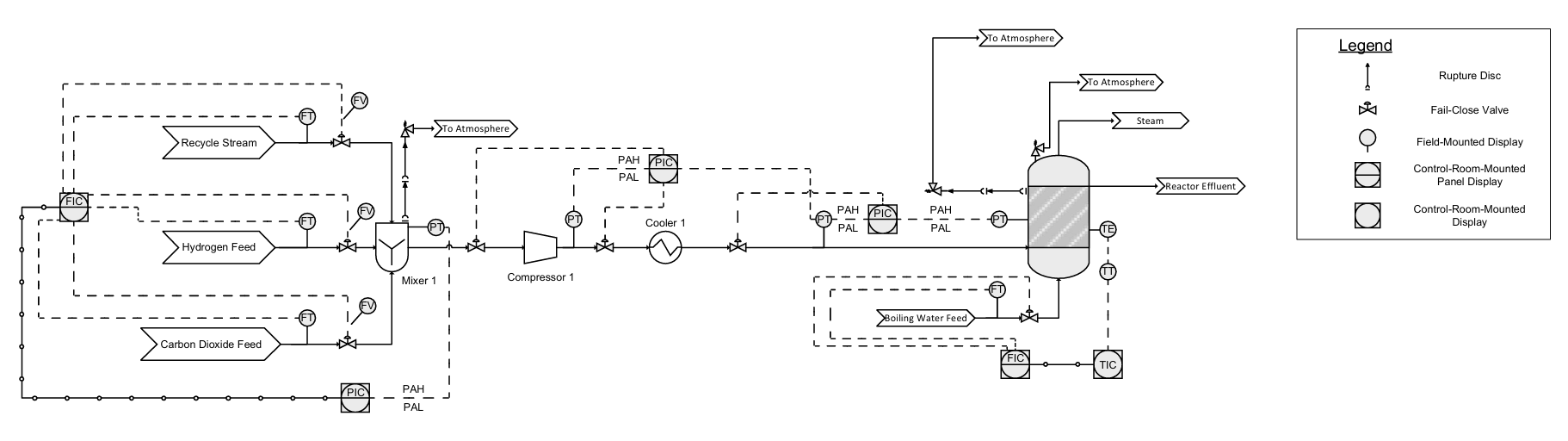
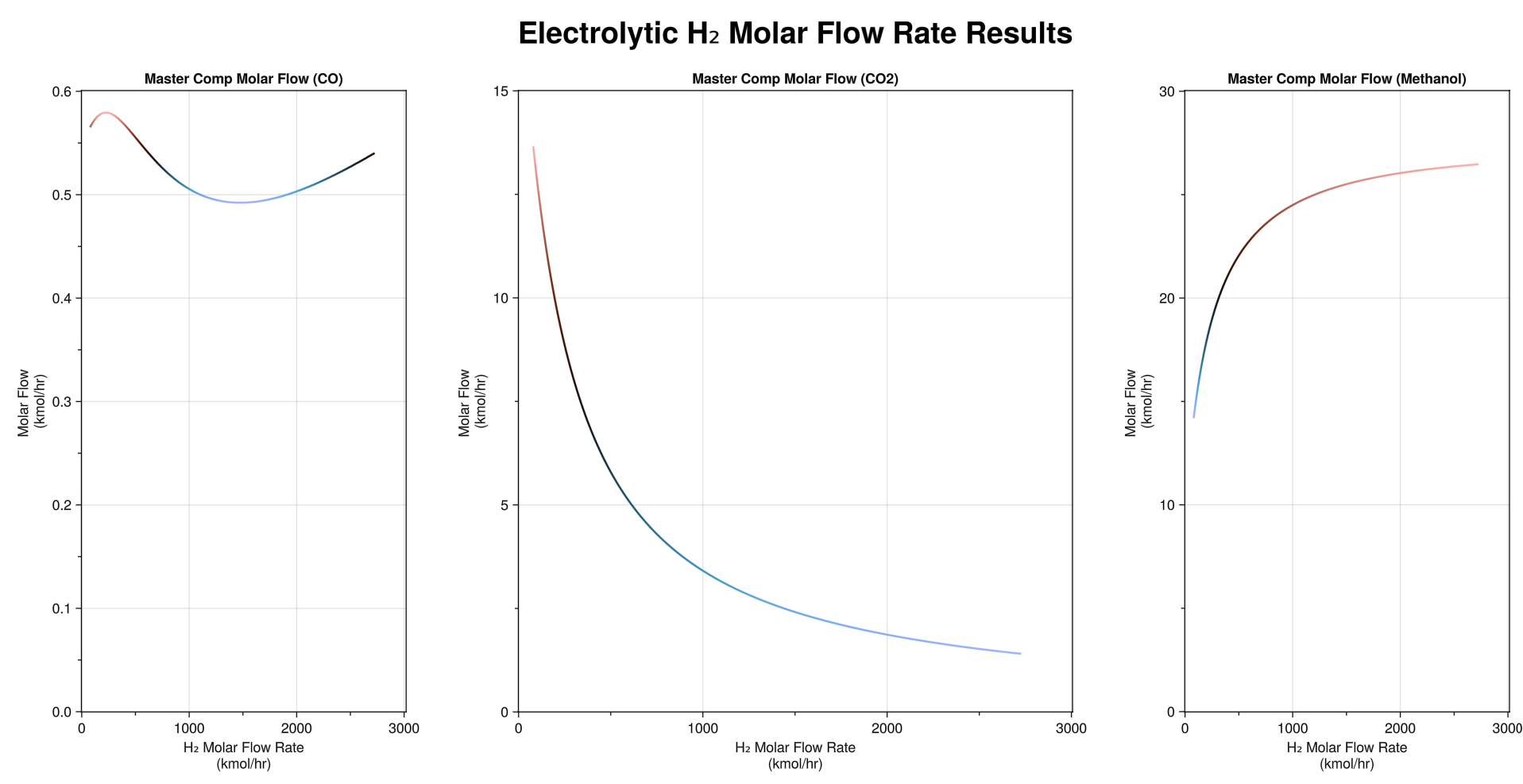
(site development, process and chemistry research, process development, ASPEN HYSYS implementation, case study optimization and visualization, hazards and operability studies, P&ID drafting, and pressure relief system design)

(project management / lead, communications, market research, equipment sizing & pricing, economic & market analysis, P&ID drafting)